Synonyms:
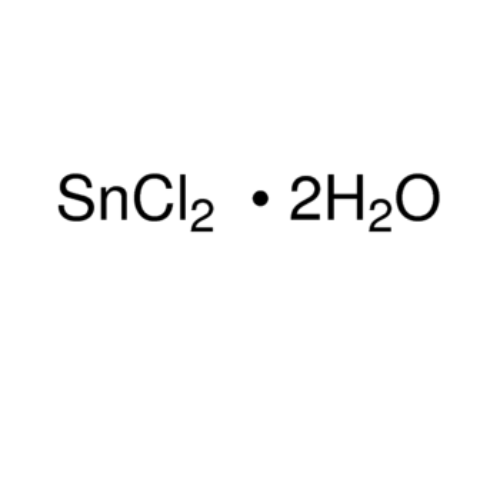
Tin (II) Chloride Dihydrate
Cas No. 10025-69-1
Molecular Weight: 225.63
Description:
Stannous Chloride is an inorganic compound that consists of tin (Sn) and chlorine (Cl). It is typically a white or colorless solid, though it can sometimes appear as a pale yellowish powder, depending on impurities and moisture content.
Properties:
- Solubility: Soluble in water and alcohol; insoluble in ether
- Hygroscopic: It tends to absorb moisture from the air, which can cause it to hydrolyze to form tin(II) hydroxide.
- Stability: Stannous chloride is relatively stable under standard conditions, but it can degrade in the presence of oxygen, turning into stannic chloride (SnCl₄) a more oxidized form.
Uses:
- Reducing Agent: Stannous chloride is commonly used as a reducing agent in various chemical reactions, particularly in organic synthesis and industrial processes. It can reduce other metal ions and compounds.
- Electroplating: It’s used in the electroplating of tin and as a component in soldering fluxes.
- Analytical Chemistry: Stannous chloride is used in some types of chemical analyses, particularly in the determination of certain metal ions by precipitation.
- Photography: It can be used in the preparation of photographic solutions as it helps in the reduction of silver salts.
- Food Industry: It is sometimes used in the food industry as a stabilizing agent in canned goods.
Safety Considerations:
- Toxicity: Stannous chloride is not highly toxic, but it should still be handled with care. Prolonged exposure or ingestion may lead to health issues.
- Storage: It should be stored in a tightly sealed container in a cool, dry place to avoid it from absorbing moisture from the air and decomposing.
Specification:
Purity: 97%
Color: White
Form: Crystalline powder


Reviews
There are no reviews yet.