Cas No. 547-58-0
Molecular Weight: 327.33
Description:
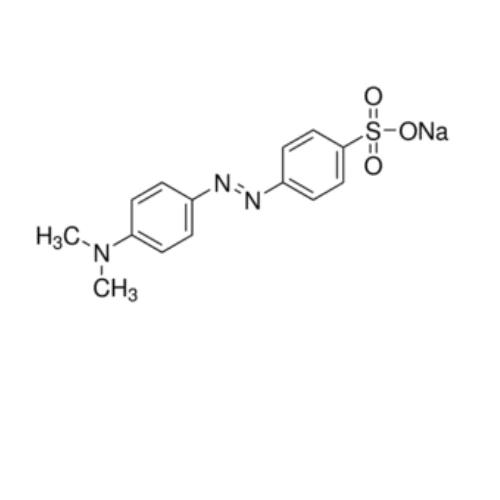
Methyl orange is a synthetic dye commonly used as a pH indicator in titrations, particularly in acid-base reactions. It is an azo dye, which means it contains a nitrogen-nitrogen double bond (–N=N–) linking aromatic rings.
Here are some key characteristics of methyl orange:
- Chemical Structure: It’s a sodium salt of the azo compound.
- Color Change: Methyl orange changes color depending on the pH of the solution:
- In acidic solutions (pH < 3.4), it appears red.
- In alkaline solutions (pH > 4.4), it turns yellow.
The color change occurs over the pH range of approximately 3.4 to 4.4, making it suitable for detecting weak acid/strong base and strong acid/weak base titrations.
- Uses:
- It is used in titrations to determine the endpoint of acid-base reactions.
- Also used as a dye in the textile industry and in various scientific applications.
- Safety: While methyl orange is not highly toxic, it can cause skin or eye irritation, and care should be taken when handling it.
Specification:
Color: Orange
Form: Crystalline powder
Loss on dryingmax. 5%
Absorbance (A) of 1% solution (pH 4.4) in a
1cm cell
@470nm750 – 850
pH range3.1 (Pink orange) to 4.4 (Yellow)


Reviews
There are no reviews yet.